Toxoplasmosis
Toxoplasmosis is contagious disease of swine, sheep and other species characterized with encephalitis, pneumonia and neonatal mortality. It is caused by protozoon Toxoplasma gondii in animals and humans. Toxoplasma is most frequently found in pigs and sheep. Young animals are infected to a lesser degree than old animals. Cattle are rarely affected with clinical toxoplasmosis. Young pigs may die from pneumonia caused by toxoplasmosis.
Humans can get infected with Toxoplasma cysts by ingestion of uncooked animal tissue. In humans clinical symptoms may vary from fever, malaise, skin rash, pneumonia, myocarditis, lymphadenopathy and encephalitis. Infected pregnant women may transfer the tachyzoites to the fetus. Toxoplasmosis has been determined to be the cause of behavioral disturbances according to one study. The neurotropic protozoan parasite toxoplasma gone is one of the main candidates and has been associated with many psychiatric disorders, including schizophrenia, claims scientists writing for the Folia Parasitologica Report. These studies have conclusively demonstrated behavioral changes, mood changes, personality changes, and cognitive impairments related to this parasite in both animal models and controlled human research studies.
Clinical Signs
Weaned pigs fed oocysts or tissue cysts generally developed weight loss, anorexia, fever, but generally recovered by three weeks p.i., irrespective of the T. gondii isolate.
A review of published data suggested that it is difficult to produce congenital toxoplasmosis consistently in swine (Dubey and Beattie, 1988). The reasons for failure and success are not well understood. The stage of T. gondii, route of inoculation, stage of gestation, and breed of sow may account for some of these variations. Some of these factors are reviewed.
Conclusion
Review of literature indicates that pigs can be raised in confinement T. gondii-free if there is adequate rodent control and contamination of feed and water with oocysts can be prevented. More research is needed to define conditions for controlling T. gondii infection in free-range or organically raised pigs. Studies indicate that pumping certain salts in pork to make it more tender or improve its shelf life kills T. gondii. Further research is needed to standardize conditions (curing, salting) for killing T. gondii in processed pork. There is a need to improve antigens for ELISA for the detection of T. gondii antibodies in pigs.
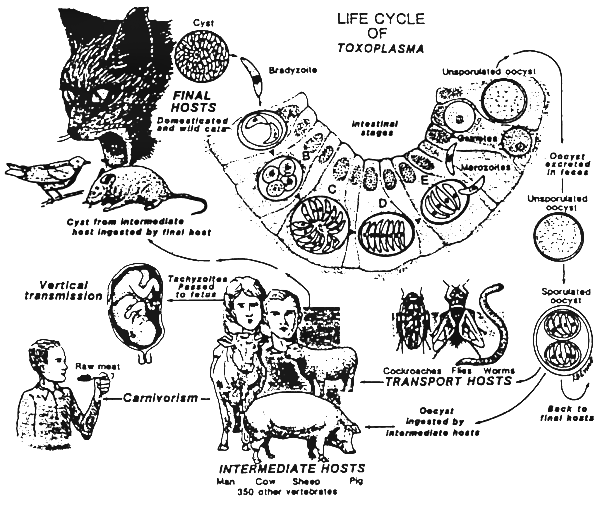
Life cycle: Asexual, sexual and oocyst stages of this organism develop in the small intestine of wild and domestic cats. Cats get infected by eating mice or birds or animal tissue containing infective oocysts. In the intestine, the parasite develops through the typical coccidian life cycle. Unsporulated oocysts are shed in the faeces. After a few days the oocysts sporulate and become infective for over a year. The oocysts are further ingested by the intermediate host (pig, sheep, cattle and humans). From the intestine, oocysts move to various tissues including myocardium, lungs, placenta and most frequently to muscle, brain and liver where they encyst. In the host, they may remain viable for the life span of the host. By eating the infected tissue mice, birds, cats and humans may get infected. The life cycle is then completed.
Antemortem findings:
Postmortem findings:
Differential diagnosis: Abortion in pigs: Brucellosis, leptospirosis, porcine parvovirus infection, hog cholera and Aujeszky's disease. Encephalitis: Salt poisoning, chlorinated hydrocarbons, lead, mercury, Vitamin A deficiency, hypoglycaemia, encephalomalacia, meningitis, rabies and scrapie
This parasite also causes mortality in pigs, especially neonatal pigs. Most pigs acquire T. gondii infection postnatally by ingestion of oocysts from contaminated environment or ingestion of infected tissues of animals. Few pigs become infected prenatally by transplacental transmission of the parasite. Raising pigs indoors in confinement has greatly reduced T. gondii infection in pigs but the recent trend of organic farming is likely to increase T. gondii infection in pigs. Recently, feeding goat whey to pigs was found to be a risk factor for T. gondii infection in organically raised pigs. Currently used molecular and histopathological methods are insensitive for the detection of T. gondii in pork because of the low concentration of the parasite in meat destined for human consumption. There is no vaccine to prevent T. gondii infection in pigs but efforts are being continued to develop a non-viable vaccine. In the present paper, information on prevalence, transmission, diagnosis, and control of porcine toxoplasmosis in the last 20 years (since 1988 when last reviewed by this author) is reviewed. Worldwide reports of clinical and asymptomatic infections in pigs are reviewed. Methods to detect T. gondii in pigs are compared. Recent studies on genetic typing of T. gondii strains prevalent in pigs are discussed with respect to epidemiology. Because wild pigs are hunted for food for human consumption prevalence in wild pigs is summarized.
Severe clinical toxoplasmosis in pigs is considered rare and reports prior to 1988 were summarized by Dubey (1986) and Dubey and Beattie (1988). Since 1988, there have been reports of clinical toxoplasmosis in weaned pigs (Weissenbo ̈ ck and Dubey, 1993; Okamoto et al., 1989; Liao et al., 2006) and in neonatal pigs (Kumagai et al., 1988; Haritani et al., 1988; Chang et al., 1990b; Giraldi et al., 1996; Thiptara et al., 2006).
Okamoto et al. (1989) reported simultaneous out- breaks of acute toxoplasmosis on five farms in Japan, perhaps associated with feed contaminated with oocysts. On a farm in Austria, 13 of 80 feeder pigs became ill (Weissenbo ̈ck and Dubey, 1993). Clinical signs included anorexia, fever, dyspnea, limb weakness, and seven died. Three pigs that died and two surviving pigs were examined histopathologically. T. gondii was found in lesions and the diagnosis was confirmed immunohisto- chemically. All seven pigs had high dye test titers (1: 65536). Epidemiological investigation suggested that pigs probably became infected by ingesting food contaminated with oocysts (Weissenbo ̈ck and Dubey, 1993). Liao et al. (2006) reported clinical toxoplasmosis in two sows from People’s Republic of China. Both sows died. T. gondii tachyzoites were seen microscopically in smear made from bronchopulmonary lymph node of sow 1 but not sow no. 2. However, T. gondii was isolated from tissues of both sows by bioassays in mice, and the diagnosis was confirmed by PCR.
Kumagai et al. (1988) reported neonatal toxoplasmosis in pigs. Seven piglets were born to one of seven sows on a farm in Japan, four of these piglets were stillborn. Three of the seven piglets had gait abnormalities and died before they were 15 days old. On necropsy, the piglet had evidence of encephalitis, pneumonitis, and lymph node necrosis. The diagnosis was confirmed immunohisto-chemically, and by finding antibodies to T. gondii (Kumagai et al., 1988).
Haritani et al. (1988) reported congenital toxoplasmo- sis in four stillborn and one live born piglet in Japan. Encephalitis and T. gondii was identified in all five piglets. Four of these animals also had pneumonia, and two had hepatic necrosis.
Chang et al. (1990b) diagnosed toxoplasmosis abortion in 20 of 120 swine on eight farms in Taiwan during June 1987 to July 1988. Degenerative changes associated with tachyzoites were found in placenta and fetal tissues, but they did not provide quantitative data.
Neonatal toxoplasmosis in pigs in Brazil was reported by Giraldi et al. (1996). They found T. gondii in tissues of two aborted fetuses, six stillborn, and 10 neonatal piglets; in histological sections of brains from 15 piglets, hearts of 13 piglets, lungs of 12 piglets, livers of 11 piglets, retinas of 10 piglets and spleens of five piglets.
Pigs can be infected from a variety of sources, including wild and domestic animals in the immediate proximity of pig barns. Lehmann et al. (2003) used genetic and ecological methods to study transmission on a pig farm in the USA. They isolated viable T. gondii from pigs and other domestic and wild animals on the farm and genetically compared isolates from pigs and from animals at different locations on the farm. The results indicated that the transmission of T. gondii was higher near the pig sties than in the surroundings in terms of strain composi- tion and risk of infection. They concluded that T. gondii infection in pigs most likely originated in the pig barn and not from outside the barn.
Recently, Villari et al. (2009) found that origin of pigs (foreign versus born locally), age of the pigs, management, number of pigs on the farm, and source of water were the main factors for T. gondii seroprevalence in pigs in Sicily, Italy. Seroprevalence in market age pigs was 7% and the highest than in neighbouring countries. The altitude of the farm and the number of animals were also important risk factors; seroprevalence was lower on farms at higher altitudes (>200 meters) and on farms with less than 50 pigs on the farm.
Using the 2006 NAHMS data, Hill et al. (2009) found that rodent control and carcass disposal methods affected the seroprevalence in pigs on 185 swine production facilities in 16 states of the US. Burial or composting of dead pigs on the farm was a risk factor for increased seroprevalence as was the lack of professional rodent control services on the farm.
Source: http://www.fao.org
https://naldc.nal.usda.gov/toxoplasmosis/PDF
Humans can get infected with Toxoplasma cysts by ingestion of uncooked animal tissue. In humans clinical symptoms may vary from fever, malaise, skin rash, pneumonia, myocarditis, lymphadenopathy and encephalitis. Infected pregnant women may transfer the tachyzoites to the fetus. Toxoplasmosis has been determined to be the cause of behavioral disturbances according to one study. The neurotropic protozoan parasite toxoplasma gone is one of the main candidates and has been associated with many psychiatric disorders, including schizophrenia, claims scientists writing for the Folia Parasitologica Report. These studies have conclusively demonstrated behavioral changes, mood changes, personality changes, and cognitive impairments related to this parasite in both animal models and controlled human research studies.
Clinical Signs
Weaned pigs fed oocysts or tissue cysts generally developed weight loss, anorexia, fever, but generally recovered by three weeks p.i., irrespective of the T. gondii isolate.
A review of published data suggested that it is difficult to produce congenital toxoplasmosis consistently in swine (Dubey and Beattie, 1988). The reasons for failure and success are not well understood. The stage of T. gondii, route of inoculation, stage of gestation, and breed of sow may account for some of these variations. Some of these factors are reviewed.
Conclusion
Review of literature indicates that pigs can be raised in confinement T. gondii-free if there is adequate rodent control and contamination of feed and water with oocysts can be prevented. More research is needed to define conditions for controlling T. gondii infection in free-range or organically raised pigs. Studies indicate that pumping certain salts in pork to make it more tender or improve its shelf life kills T. gondii. Further research is needed to standardize conditions (curing, salting) for killing T. gondii in processed pork. There is a need to improve antigens for ELISA for the detection of T. gondii antibodies in pigs.
Life cycle: Asexual, sexual and oocyst stages of this organism develop in the small intestine of wild and domestic cats. Cats get infected by eating mice or birds or animal tissue containing infective oocysts. In the intestine, the parasite develops through the typical coccidian life cycle. Unsporulated oocysts are shed in the faeces. After a few days the oocysts sporulate and become infective for over a year. The oocysts are further ingested by the intermediate host (pig, sheep, cattle and humans). From the intestine, oocysts move to various tissues including myocardium, lungs, placenta and most frequently to muscle, brain and liver where they encyst. In the host, they may remain viable for the life span of the host. By eating the infected tissue mice, birds, cats and humans may get infected. The life cycle is then completed.
Antemortem findings:
- Neonatal mortality
- Fever (40 – 42°C) and pneumonia in young pigs
- Difficult breathing and coughing
- Weakness and wasting
- Incoordination and trembling
- Diarrhoea
- Abortion in pregnant sows and stillbirths
Postmortem findings:
- neumonia
- Hydrothorax
- Ascites
- Intestinal ulceration
- Necrosis in the liver, spleen and kidneys
- Inflammation of the lymph nodes
- Multiple granulomatous lesion in the brain
Differential diagnosis: Abortion in pigs: Brucellosis, leptospirosis, porcine parvovirus infection, hog cholera and Aujeszky's disease. Encephalitis: Salt poisoning, chlorinated hydrocarbons, lead, mercury, Vitamin A deficiency, hypoglycaemia, encephalomalacia, meningitis, rabies and scrapie
This parasite also causes mortality in pigs, especially neonatal pigs. Most pigs acquire T. gondii infection postnatally by ingestion of oocysts from contaminated environment or ingestion of infected tissues of animals. Few pigs become infected prenatally by transplacental transmission of the parasite. Raising pigs indoors in confinement has greatly reduced T. gondii infection in pigs but the recent trend of organic farming is likely to increase T. gondii infection in pigs. Recently, feeding goat whey to pigs was found to be a risk factor for T. gondii infection in organically raised pigs. Currently used molecular and histopathological methods are insensitive for the detection of T. gondii in pork because of the low concentration of the parasite in meat destined for human consumption. There is no vaccine to prevent T. gondii infection in pigs but efforts are being continued to develop a non-viable vaccine. In the present paper, information on prevalence, transmission, diagnosis, and control of porcine toxoplasmosis in the last 20 years (since 1988 when last reviewed by this author) is reviewed. Worldwide reports of clinical and asymptomatic infections in pigs are reviewed. Methods to detect T. gondii in pigs are compared. Recent studies on genetic typing of T. gondii strains prevalent in pigs are discussed with respect to epidemiology. Because wild pigs are hunted for food for human consumption prevalence in wild pigs is summarized.
Severe clinical toxoplasmosis in pigs is considered rare and reports prior to 1988 were summarized by Dubey (1986) and Dubey and Beattie (1988). Since 1988, there have been reports of clinical toxoplasmosis in weaned pigs (Weissenbo ̈ ck and Dubey, 1993; Okamoto et al., 1989; Liao et al., 2006) and in neonatal pigs (Kumagai et al., 1988; Haritani et al., 1988; Chang et al., 1990b; Giraldi et al., 1996; Thiptara et al., 2006).
Okamoto et al. (1989) reported simultaneous out- breaks of acute toxoplasmosis on five farms in Japan, perhaps associated with feed contaminated with oocysts. On a farm in Austria, 13 of 80 feeder pigs became ill (Weissenbo ̈ck and Dubey, 1993). Clinical signs included anorexia, fever, dyspnea, limb weakness, and seven died. Three pigs that died and two surviving pigs were examined histopathologically. T. gondii was found in lesions and the diagnosis was confirmed immunohisto- chemically. All seven pigs had high dye test titers (1: 65536). Epidemiological investigation suggested that pigs probably became infected by ingesting food contaminated with oocysts (Weissenbo ̈ck and Dubey, 1993). Liao et al. (2006) reported clinical toxoplasmosis in two sows from People’s Republic of China. Both sows died. T. gondii tachyzoites were seen microscopically in smear made from bronchopulmonary lymph node of sow 1 but not sow no. 2. However, T. gondii was isolated from tissues of both sows by bioassays in mice, and the diagnosis was confirmed by PCR.
Kumagai et al. (1988) reported neonatal toxoplasmosis in pigs. Seven piglets were born to one of seven sows on a farm in Japan, four of these piglets were stillborn. Three of the seven piglets had gait abnormalities and died before they were 15 days old. On necropsy, the piglet had evidence of encephalitis, pneumonitis, and lymph node necrosis. The diagnosis was confirmed immunohisto-chemically, and by finding antibodies to T. gondii (Kumagai et al., 1988).
Haritani et al. (1988) reported congenital toxoplasmo- sis in four stillborn and one live born piglet in Japan. Encephalitis and T. gondii was identified in all five piglets. Four of these animals also had pneumonia, and two had hepatic necrosis.
Chang et al. (1990b) diagnosed toxoplasmosis abortion in 20 of 120 swine on eight farms in Taiwan during June 1987 to July 1988. Degenerative changes associated with tachyzoites were found in placenta and fetal tissues, but they did not provide quantitative data.
Neonatal toxoplasmosis in pigs in Brazil was reported by Giraldi et al. (1996). They found T. gondii in tissues of two aborted fetuses, six stillborn, and 10 neonatal piglets; in histological sections of brains from 15 piglets, hearts of 13 piglets, lungs of 12 piglets, livers of 11 piglets, retinas of 10 piglets and spleens of five piglets.
Pigs can be infected from a variety of sources, including wild and domestic animals in the immediate proximity of pig barns. Lehmann et al. (2003) used genetic and ecological methods to study transmission on a pig farm in the USA. They isolated viable T. gondii from pigs and other domestic and wild animals on the farm and genetically compared isolates from pigs and from animals at different locations on the farm. The results indicated that the transmission of T. gondii was higher near the pig sties than in the surroundings in terms of strain composi- tion and risk of infection. They concluded that T. gondii infection in pigs most likely originated in the pig barn and not from outside the barn.
Recently, Villari et al. (2009) found that origin of pigs (foreign versus born locally), age of the pigs, management, number of pigs on the farm, and source of water were the main factors for T. gondii seroprevalence in pigs in Sicily, Italy. Seroprevalence in market age pigs was 7% and the highest than in neighbouring countries. The altitude of the farm and the number of animals were also important risk factors; seroprevalence was lower on farms at higher altitudes (>200 meters) and on farms with less than 50 pigs on the farm.
Using the 2006 NAHMS data, Hill et al. (2009) found that rodent control and carcass disposal methods affected the seroprevalence in pigs on 185 swine production facilities in 16 states of the US. Burial or composting of dead pigs on the farm was a risk factor for increased seroprevalence as was the lack of professional rodent control services on the farm.
Source: http://www.fao.org
https://naldc.nal.usda.gov/toxoplasmosis/PDF